Abstract
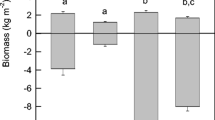
Several studies addressed aboveground biomass recovery in tropical and subtropical seagrass systems following physical disturbance. However, there are few studies documenting belowground biomass recovery despite the important functional and ecological role of roots and rhizomes for seagrass ecosystems. In this study, we compared the recovery of biomass (g dry weight m−2) as well as the biomass recovery rates in ten severely disturbed multi-species seagrass meadows, after the sediments were excavated and the seagrasses removed. Three sites were located in the tropics (Puerto Rico) and seven in the subtropics (Florida Keys), and all were originally dominated by Thalassia testudinum. Total aboveground biomass reached reference values at four out of ten sites studied, two in the Florida Keys and two in Puerto Rico. Total belowground biomass was lower at the disturbed locations compared to the references at all sites, apart from two sites in the Florida Keys where the compensatory effect of opportunistic species (Syringodium filiforme and Halodule wrightii) was observed. The results revealed large variation among sites in aboveground and belowground biomass for all species, with higher aboveground recovery than belowground for T. testudinum. Recovery rates for T. testudinum were highly variable across sites, but a general trend of faster aboveground than belowground recovery was observed. Equal rates between aboveground and belowground biomass were found for opportunistic species at several sites in the Florida Keys. These results indicate the importance of belowground biomass when assessing seagrass recovery and suggest that the appropriate metric to assess seagrass recovery should address belowground biomass as well as aboveground biomass in order to evaluate the full recovery of ecological services and functions performed by seagrasses. We point out regional differences in species composition and species shifts following severe disturbance events and discuss ecological implications of gap dynamics in multi-species seagrass meadows.





Similar content being viewed by others
References
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecol 26:32–46
Bell SB, Robbins BD, Jensen SL (1999) Gap dynamics in a seagrass landscape. Ecosystems 2:493–504
Bell SB, Fonseca MS, Stafford NB (2006) Seagrass ecology: new contributions from a landscape perspective. In: Larkum AW, Duarte CM, Orth RJ (eds) Seagrass biology. Springer, Netherlands, pp 625–645
Braun-Blanquet J (1932) Plant sociology—the study of plant communities. Koeltz Scientific Books, Koenigstein
Costanza R, d’Arge R, de Groot R, Farber S, Grasso M, Hannon B, Limburg K, Naeem S, O’Neill RV, Paruelo J, Raskin RG, Sutton P, van den Belt M (1997) The value of the world’s ecosystem services and natural capital. Nature 387:253–260
Dawes CJ, Andorfer J, Rose C, Uranowski C, Ehringer N (1997) Regrowth of Thalassia testudinum into propeller scars. Aquat Bot 59:139–155
de Boer WF (2007) Seagrass–sediment interactions, positive feedbacks and critical thresholds for occurrence: a review. Hydrobiologia 591:5–24
den Hartog C (1970) The seagrasses of the world. North Holland Publishing, The Netherlands
den Hartog C (1971) The dynamic aspect in the ecology of sea-grass communities. Thal Jugo 7:101–112
Duarte CM (1991) Allometric scaling of seagrass form and productivity. Mar Ecol Prog Ser 77:289–300
Duarte CM (2000) Marine biodiversity and ecosystem services: an elusive link. J Exp Mar Biol Ecol 250:117–131
Duarte CM, Cebrian J (1996) The fate of marine autotrophic production. Limnol Oceanogr 41:1758–1766
Duarte CM, Chiscano CL (1999) Seagrass biomass and production: a reassessment. Aquat Bot 65:159–174
Duarte CM, Merino M, Agawin NSR, Uri J, Fortes MD, Gallegos ME, Marba’ N, Hemminga M (1998) Root production and belowground seagrass biomass. Mar Ecol Prog Ser 171:97–108
Duarte CM, Fourqurean JW, Krause-Jensen D, Olesen B (2006) Dynamics of seagrasses. In: Larkum AW, Duarte CM, Orth RJ (eds) Seagrass biology. Springer, Netherlands, pp 271–294
Duffy JE (2006) Biodiversity and the functioning of seagrass ecosystems. Mar Ecol Prog Ser 311:233–250
Durako MJ, Hall MO, Merello M (2002) Patterns of change in the seagrass dominated Florida Bay hydroscape. In: Porter JW, Porter KG (eds) The Everglades, Florida Bay and Coral Reefs of the Florid Keys: an ecosystem sourcebook. CRC, Boca Raton, pp 523–537
Fonseca MS, Bell SS (1998) The influence of wave exposure and tidal currents on seagrass landscapes. Mar Ecol Prog Ser 171:109–121
Fonseca MS, Fisher JS (1986) A comparison of canopy friction and sediment movement between four species of seagrass with reference to their ecology and restoration. Mar Ecol Prog Ser 29:15–22
Fonseca MS, Julius BE, Kenworthy WJ (2000) Integrating biology and economics in seagrass restoration: how much is enough and why? Ecol Eng 15:227–237
Fourqurean JW, Powell GVN, Kenworthy WJ, Zieman JC (1995) The effects of long-term manipulation of nutrient supply on competition between the seagrasses Thalassia testudinum and Halodule wrightii in Florida Bay. Oikos 72:349–358
Fourqurean JW, Willsie A, Rose CD, Rutten LM (2001) Spatial and temporal patterns in seagrass community composition and productivity in south Florida. Mar Biol 138:341–354
Gallegos ME, Merino M, Marba N, Duarte CM (1993) Biomass and dynamics of Thalassia testudinum in the Mexican Caribbean: elucidating rhizome growth. Mar Ecol Prog Ser 95:185–192
Gallegos ME, Merino M, Rodriguez A, Marba’ N, Duarte CM (1994) Growth patterns and demography of pioneer Caribbean seagrasses Halodule wrightii and Syringodium filiforme. Mar Ecol Prog Ser 109:99–104
Ginsburg RN, Lowenstam HA (1958) The influence of marine bottom communities on the depositional environment of sediments. J Geol 66:310–318
Hall LM, Hanisak MD, Virnstein RW (2006) Fragments of the seagrasses Halodule wrightii and Halophila johnsonii as potential recruits in Indian River Lagoon, Florida. Mar Ecol Prog Ser 310:109–117
Hemminga MA (1998) The root/rhizome system of seagrasses: an asset and a burden. J Sea Res 39:183–196
Hemminga MA, Duarte CM (2000) Seagrass ecology. Cambridge University Press, Cambridge
Kaldy JE, Dunton KH (2000) Above- and belowground production, biomass and reproductive ecology of Thalassia testudinum (turtle grass) in a subtropical lagoon. Mar Ecol Prog Ser 193:271–283
Kenworthy WJ, Thayer GW (1984) Production and decomposition of the roots and rhizomes of seagrasses, Zostera marina and Thalassia testudinum, in temperate and subtropical marine ecosystems. Bull Mar Sci 35:364–379
Kenworthy WJ, Fonseca MS, Whitfield PE, Hammerstrom KK (2002) Analysis of seagrass recovery in experimental excavations and propeller-scar disturbances in the Florida Keys National Marine Sanctuary. J Coast Res SI37:75–85
Kenworthy WJ, Wyllie-Echeverria S, Coles RG, Pergent G, Pergent-Martini C (2006) Seagrass conservation biology: an interdisciplinary science for protection of the seagrass biome. In: Larkum AW, Duarte CM, Orth RJ (eds) Seagrass biology. Springer, Netherlands, pp 595–623
Kirsch K, Barry KA, Fonseca MS, Whitfield PE, Meehan SR, Kenworthy WJ, Julius BE (2005) The Mini-312 Program-an expedited damage assessment and restoration process for seagrasses in the Florida Keys National Marine Sanctuary. J Coast Res SI40:109–119
Kuo J, den Hartog C (2006) Seagrass morphology, anatomy, and ultrastructure. In: Larkum AWD, Orth RJ, Duarte CM (eds) Seagrasses: biology, ecology and conservation. Springer, Dordrecht, pp 51–87
Lee KS, Dunton KH (1999) Influence of sediment nitrogen availability on carbon and nitrogen dynamics in the seagrass Thalassia testudinum. Mar Biol 134:217–226
Lefebvre LW, Reid JW, Kenworthy WJ, Powell JA (2000) Characterizing Manatee habitat use in Florida and Puerto Rico: implications for conservation and management. Pac Conserv Biol 5:289–298
Marba’ N, Duarte CM (2001) Growth and sediment space occupation by seagrass Cymodocea nodosa roots. Mar Ecol Prog Ser 224:291–298
Mateo MA, Romero J, Perez M, Littler MM, Littler DS (1997) Dynamics of millenary organic deposits resulting from the growth of the Mediterranean seagrass Posidonia oceanica. Estuar Coast Shelf Sci 44:103–110
McArdle BH, Anderson MJ (2001) Fitting multivariate models to community data: a comment on distance based redundancy analysis. Ecology 82:290–297
Neckles HA (1994) Indicator development: seagrass monitoring and research in the Gulf of Mexico. U.S. Environmental Protection Agency, Office of Research and Development, Environmental Research Laboratory, Gulf Breeze, FL. EPA/620/R-94/-29
Olesen B, Marba’ N, Duarte CM, Savela RS, Fortes MD (2004) Recolonization dynamics in a mixed seagrass meadow: the role of clonal versus sexual processes. Estuaries 27:770–780
Onuf CP (2000) Seagrass response to and recovery from seven years of brown tide. Pac Conserv Biol 5:306–313
Orth RJ, Carruthers TJB, Dennison WC, Duarte CM, Fourqurean JW, Heck KL Jr, Hughes AR, Hendrick GA, Kenworthy WJ, Olyarnik S, Short FT, Waycott M, Williams SL (2006) A global crisis for seagrass ecosystems. Bioscience 56:987–996
Parker KR, Wiens JA (2005) Assessing recovery following environmental accidents: environmental variation, ecological assumptions, and strategies. Ecol Appl 15:2037–2051
Patriquin DG (1975) Migration of “blowouts” in seagrass beds at Barbados and Carriaco, West Indes and its ecological and geological implications. Aquat Bot 1:163–189
Peterson BJ, Rose CR, Rutten LM, Fourqurean JW (2002) Disturbance and recovery following catastrophic grazing: studies of a successional chronosequence in seagrass bed. Oikos 97:361–370
Rasheed MA (1999) Recovery of experimentally created gaps within a tropical Zostera capricorni (Aschers.) seagrass meadow, Queensland Australia. J Exp Mar Biol Ecol 235:183–200
Rollon RN, Van Steveninck EDDR, Vierssen WV, Fortes MD (1998) Contrasting recolonization strategies in multi-species seagrass meadows. Mar Poll Bull 37:450–459
Skilleter GA, Cameron B, Zharikov Y, Boland D, McPhee DP (2006) Effects of physical disturbance on infaunal and epifaunal assemblages in subtropical, intertidal seagrass beds. Mar Ecol Prog Ser 308:61–78
Thayer GW, Kenworthy WJ, Fonseca MS (1984) The ecology of eelgrass meadows of the Atlantic coast: a community profile. U.S. Fish Wildlife Service FWS/OBS-84/02, Washington, DC
Thayer GW, Murphy PL, LaCroix MW (1994) Responses of plant communities in western Florida Bay to the die-off of seagrasses. Bull Mar Sci 54:718–726
Wanless HR, Tagett MG (1989) Origin, growth and evolution of carbonate mudbanks in Florida Bay. Bull Mar Sci 44:454–489
Whitfield PE, Kenworthy WJ, Hammerstrom KK, Fonseca MS (2002) The role of storms in the expansion of disturbances initiated by motor vessels on subtropical seagrass-coral banks. J Coast Res SI37:86–99
Williams SL (1990) Experimental studies of Caribbean seagrass bed development. Ecol Monogr 60:449–469
Zieman JC (1982) The ecology of the seagrasses of south Florida: a community profile. U.S. Fish Wildlife Service, FWS/OBS82/25, Washington, DC
Acknowledgments
The authors wish to acknowledge the officers and crew of NOAA ship NANCY FOSTER for their professional assistance during the cruises and for the good time while onboard. Research cruises require a great deal of work and preparation and we are grateful to the large number of scientists that made this project possible. In particular, we would like to thank Manuel Merello, Sean Meehan, Amy Uhrin, Shay Viehman, Lisa Wood, Brooke Landry, Jennifer Kunzelman, John Hackney, Penny Hall, Donna Berns, Kevin Kirsch, Brian Degan, Kevin Madley, Mark Julian, Erika Hansen and Jim Reid. We are also grateful to all those people that helped us process the many seagrass samples; thanks go to the Marine Mammal Group at the NOAA Beaufort Laboratory (Emma, Patti, Gretchen, Annie), Mike Lacroix, Patricia Hay, Gary Fisher, Brad Teer, Jenny VanderPlum and Vanessa Nero. We are indebted to Ray Gorley for his endless patience and his invaluable support with PRIMER and PERMANOVA software. Useful comments on the statistics and the text were provided by Antonio Terlizzi, Marti Jane Anderson, Shay Viehman, Lisandro Benedetti-Cecchi, Mike J. Durako and three anonymous reviewers. This project was funded by a combination of sources including: Programmatic funds provided by the Center for Coastal Fishery and Habitat Research (CCFHR), National Centers for Coastal Ocean Science (NCCOS), National Ocean Service, NOAA, a Long-Term Agreement between NCCOS and the Office of National Marine Sanctuaries, NOAA’s Office of Response and Restoration, and the Florida Fish and Wildlife Research Commission. Giuseppe Di Carlos’s Post-Doctoral Fellowship at CCFHR was supported by the National Research Council/National Academies of Science.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Martin Attrill.
Electronic supplementary material
Below is the link to the electronic supplementary material.
442_2008_1120_MOESM1_ESM.eps
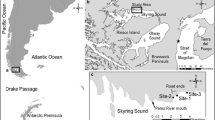
Fig. S1 Study sites location. Circles in the Caribbean Sea map correspond to enlarged areas of the Florida Keys and southeastern Puerto Rico (EPS 5.80 mb)
Rights and permissions
About this article
Cite this article
Di Carlo, G., Kenworthy, W.J. Evaluation of aboveground and belowground biomass recovery in physically disturbed seagrass beds. Oecologia 158, 285–298 (2008). https://doi.org/10.1007/s00442-008-1120-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-008-1120-0